Stereospecific coupling of chiral tertiary alkyl bromide and alkyne:
Convenient synthetic method for chiral quaternary carbon center
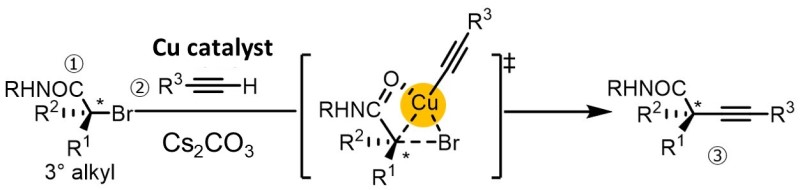
Nishikata’s group (Professor at Yamaguchi University, Graduate School of Sciences and Technology for Innovation) reports the stereospecific coupling of a chiral α-bromocarboxamide bearing a tert-alkyl moiety and an alkyne to produce a chiral tert-alkylated alkyne possessing chiral quaternary carbon center. The CuBr/bathophen was used as a catalyst, and a carboxamide group was used as the directing group. This reaction produced various alkynes in moderate to good yields and a retention of up to 99%.
Published Thesis Information
- Title : Carboxamide-directed Stereospecific Couplings of Chiral tertiary Alkyl Halides with Terminal Alkynes
- Authors :Akagawa, Hiroki; Tsuchiya, Naoki; Morinaga, Asuka; Katayama, Yu; Sumimoto, Michinori; NISHIKATA, Takashi
- Publication : ACS Catalysis (IF=13.7)
- Release Date : August 8, 2022 at 8 A.M. (EDT)
- D O I : 10.1021/acscatal.2c02433