Largest veterinarian-initiated multicenter clinical study of anti-PD-1 antibody in dogs with oral malignant melanoma
—Analysis of 150 cases confirms clinical activity and identifies predictive biomarkers—
Key Highlights
- A multicenter, prospective, veterinarian-initiated clinical study evaluated a caninized anti–canine PD‑1 monoclonal antibody (ca‑4F12‑E6) in 150 dogs with advanced canine oral malignant melanoma (OMM). To our knowledge, this is the largest clinical study of an anti‑PD‑1 antibody in veterinary oncology.
- Despite the aggressive and treatment‑refractory nature of advanced OMM, the best overall response rate (ORR) was 16.7%. Most adverse events were mild and manageable, supporting the feasibility of PD‑1 blockade in a large canine cohort.
- Exploratory biomarker analyses identified high microsatellite instability (MSI‑High)—a genomic signature of accumulated mutations—as a significant predictor of prolonged overall survival. This is the first large-scale evidence in canine melanoma linking MSI status to outcome following PD‑1 blockade.
- Elevated inflammatory markers on pre‑treatment blood tests (e.g., white blood cell count, C‑reactive protein [CRP]) were associated with poorer clinical response, suggesting readily accessible baseline biomarkers for patient stratification.
Background
Canine OMM is an aggressive cancer characterized by rapid local progression and a high propensity for metastasis. For dogs with advanced disease that are not amenable to definitive local therapy (surgery and/or radiotherapy), effective systemic treatment options are limited. In human medicine, immune checkpoint inhibitors—including anti‑PD‑1 antibodies—have produced transformative benefits across multiple malignancies, including melanoma. However, as of today, there is no widely available, commercially approved immune checkpoint inhibitor antibody for dogs in Japan, creating a major unmet clinical need.
Our team previously developed a caninized anti–canine PD‑1 monoclonal antibody ca‑4F12‑E6 and demonstrated feasibility in pilot studies. The next essential step was to validate efficacy and safety in a substantially larger cohort and to identify biomarkers that predict clinical benefit.
Overview
A research group led by Prof. Takuya Mizuno (Laboratory of Veterinary Clinical Pathology, Joint Faculty of Veterinary Medicine; Deputy Director, The Research Institute for Cell Design Medical Science) and Assoc. Prof. Masaya Igase (Laboratory of Veterinary Clinical Pathology, Joint Faculty of Veterinary Medicine; The Research Institute for Cell Design Medical Science), in collaboration with Dr. Kenji Hagimori (Kyoto Animal Medical Center), conducted a large-scale clinical study in dogs with advanced OMM. The study evaluated the clinical activity, safety profile, and potential predictive biomarkers of a caninized anti–canine PD‑1 monoclonal antibody (ca‑4F12‑E6), originally developed through prior efforts in collaboration with Nippon Zenyaku Kogyo Co., Ltd.
In this study, 150 client-owned dogs were enrolled and treated with intravenous ca-4F12-E6 every two weeks. Despite the advanced disease setting, an objective response—defined by measurable tumor regression—was observed in 16.7% of dogs, and a subset of responders achieved durable survival.
As a key translational component, exploratory biomarker analyses were conducted. Dogs with MSI-High tumors exhibited significantly longer overall survival compared to those with MSI-Low/microsatellite-stable (MSS) tumors (median, 200 vs. 95 days). In addition, higher baseline systemic inflammation—reflected by routine blood markers such as elevated leukocyte counts and CRP—was associated with poorer clinical outcomes.
These findings strengthen the evidence base for immune checkpoint blockade in canine oncology and support a precision‑medicine approach by enabling pre‑treatment identification of dogs most likely to benefit.
The full study was published online in the Journal for ImmunoTherapy of Cancer on January 23rd at 10:00 JST. This work was supported by the Japan Society for the Promotion of Science (JSPS) Grants‑in‑Aid for Scientific Research (KAKENHI).
※ Immune checkpoint molecules and immune checkpoint inhibitors
Immune checkpoint molecules are regulatory proteins that modulate immune responses. Representative examples include PD-1 on lymphocytes and PD-L1 expressed on tumor cells and immune cells. The mechanisms underlying immune checkpoint regulation were recognized by the 2018 Nobel Prize in Physiology. The binding of PD-1 to PD-L1 is one way by which tumor cells evade immune attack, as it suppresses the antitumor activity of tumor-infiltrating lymphocytes. Immune checkpoint inhibitor therapy blocks this interaction—typically using antibodies such as anti–PD-1 or anti–PD-L1—thereby restoring T-cell function and enhancing antitumor immunity.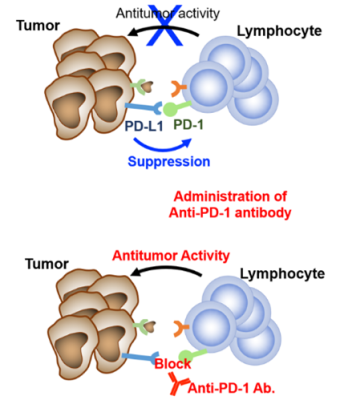
※ What is microsatellite instability?
Microsatellites are short, repetitive DNA sequences scattered throughout the genome. When DNA mismatch repair is impaired, errors accumulate during DNA replication, leading to altered microsatellite lengths (microsatellite instability). Tumors classified as MSI‑High often have high mutational burden and increased immunogenicity, which in human oncology is associated with improved responses to immune checkpoint inhibitors.
Future Perspectives
This large-scale dataset supports ca‑4F12‑E6 as a promising systemic treatment option for dogs with OMM. Importantly, MSI testing and routine pre‑treatment bloodwork may enable patient stratification and facilitate the adoption of precision medicine in veterinary oncology, ensuring that eligible dogs receive PD‑1 blockade at an optimal time point.
Yamaguchi University Animal Medical Center, Kyoto Animal Medical Center, and the Japan Small Animal Cancer Center will continue veterinarian‑initiated clinical research across multiple tumor types.
Figures
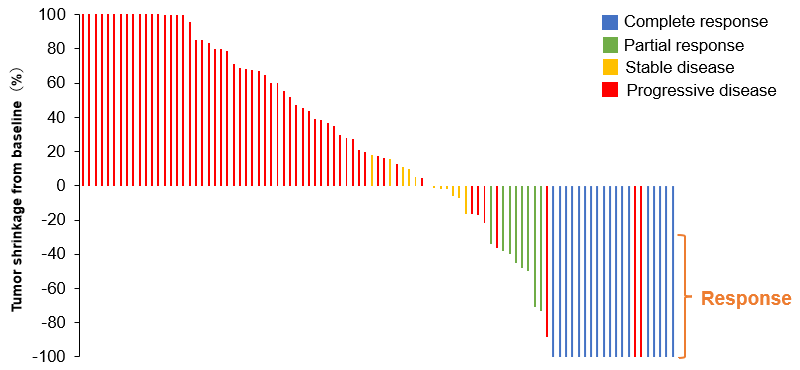
Figure 1. Tumor shrinkage from baseline following treatment
Data are shown for 95 of 150 dogs in whom tumor measurements were available before and after treatment. The best overall response rate was 16.7%.
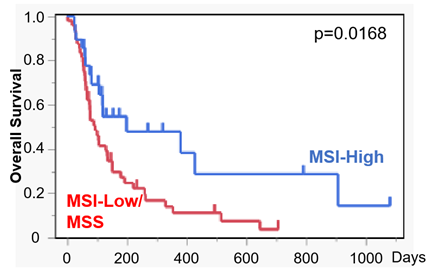
Figure 2. Overall survival according to MSI status
Overall survival was compared between dogs with MSI‑High tumors (n = 28) and those with MSI‑Low/MSS tumors (n = 48). Dogs with MSI‑High tumors showed significantly prolonged survival following treatment.
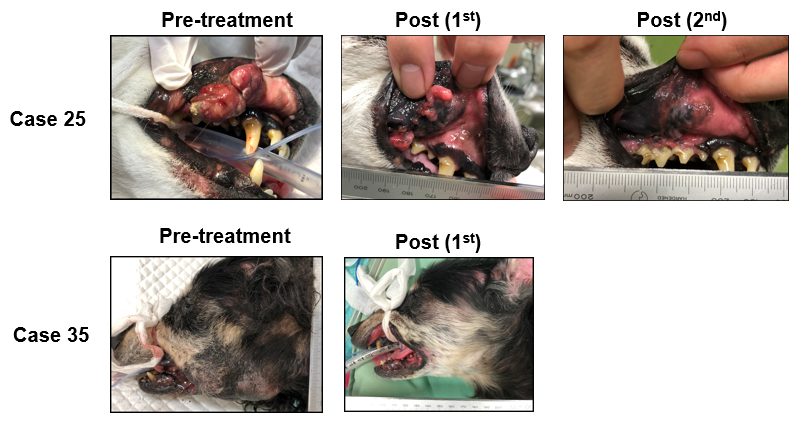
Figure 3. Representative macroscopic changes in stage IV canine oral malignant melanoma
Photographs show primary oral lesions in two stage IV cases (Case 25 and Case 35). Marked tumor regression was observed following treatment. Regression of pulmonary metastases was also observed in these cases, not shown here.
Publication Information
- Title:Caninized PD-1 monoclonal antibody in oral malignant melanoma: Efficacy and exploratory biomarker analysis
- Authors:Masaya Igase, Kenji Hagimori, Sakuya Inanaga, Hiroki Mizoguchi, Kazuhito Itamoto, Masashi Sakurai, Tomoki Motegi, Hiroka Yamamoto, Masahiro Kato, Toshinori Shiga, Toshihiro Tsukui, Tetsuya Kobayashi, Takuya Mizuno
- Journal:Journal for ImmunoTherapy of Cancer
- Publication date:January 23, 2026(Online)
- URL:https://jitc.bmj.com/lookup/doi/10.1136/jitc-2025-013623
Contact
-
Laboratory of Veterinary Clinical Pathology, Joint Faculty of Veterinary Medicine, Yamaguchi University
Division of Translational Research for One Medicine, The Research Institute for Cell Design Medical Science
1677‑1 Yoshida, Yamaguchi 753‑8515, Japan
Prof. Takuya Mizuno
Tel: +81‑83‑933‑5894
Email: mizutaku*yamaguchi-u.ac.jp (replace * with @)
Related URL: https://mizutakuvet.com/